Abstract
Objective
To compare the growth of very low birth weight preterm infants admitted to the neonatal intensive care unit, fed with pasteurized human milk and pasteurized human milk plus a commercial supplement.
Methods
This was a series of cases, with a longitudinal design. Population and sample were comprised of all the records of infants less than 36 weeks gestational age, weighing less than 1,500 grams, who met the following inclusion criteria: clinically stable, of either sex, without diseases that could interfere in the growth and weight gain, using any type of tube through which they received pasteurized human milk and/or pasteurized human milk enhanced with FM85®, a commercial fortifying additive. Two groups were created: very low birth weight infants who received pasteurized human milk (Group A) and those who received pasteurized human milk enhanced with FM85® (Group B).
Results
No statistically significant difference was found between the two groups of very low preterm newborns, with the different diets evaluated at two different times. The gestational age influenced daily weight gain in both comparisons.
Conclusion
No statistically significant difference in growth was identified when comparing the two groups.
Infant, premature; Milk, human; Milk banks; Food additives; Nursing
Resumo
Objetivo
Comparar o crescimento de recém-nascidos prematuros de muito baixo peso internados na Unidade de Terapia Intensiva Neonatal, alimentados com LHP e LHP acrescido de suplemento comercial.
Métodos
Trata-se de uma série de casos, com delineamento longitudinal. População e amostra composta por todos os prontuários de RNPTMBP com menos de 36 semanas de idade gestacional e peso menor que 1.500g que responderam aos seguintes critérios de inclusão: clinicamente estáveis, de qualquer sexo, em uso de qualquer tipo de sonda pelo qual receberam leite humano pasteurizado e/ou que receberam leite humano pasteurizado com aditivo de fortificante comercial FM85®, e, que não apresentavam patologias que pudessem interferir no crescimento e ganho de peso. A partir do levantamento, constituiu-se dois grupos: recém-nascidos pré-termo de muito baixo peso que receberam leite humano pasteurizado (Grupo A) e os que receberam leite humano pasteurizado acrescido de FM85® (Grupo B).
Resultados
verificou-se que não houve diferença estatisticamente significativa entre os recém-nascidos pré-termo de muito baixo peso avaliados na comparação entre os dois grupos e entre o grupo analisado em dois momentos com as diferentes dietas. Observou-se, que a idade gestacional influenciou no ganho de peso diário em ambas as comparações.
Conclusão
Concluiu-se que as duas dietas utilizadas não implicaram em diferença estatisticamente significativas no crescimento quando comparados os dois grupos.
Recém-nascido prematuro; Leite humano; Banco de leite; Aditivos alimentares; Enfermagem
Resumen
Objetivo
comparar el crecimiento de recién nacidos prematuros de muy bajo peso internados en la Unidad de Terapia Intensiva Neonatal, alimentados con LHP y LHP con suplemento comercial.
Métodos
se trata de una serie de casos, con delineamiento longitudinal. Población y muestra compuesta por todas las historias clínicas de los RNPT MBP con menos de 36 semanas de edad gestacional y peso menor a 1.500 g que respondían los siguientes criterios de inclusión: clínicamente estable, de cualquier sexo, en uso de cualquier tipo de sonda por la cual recibe leche humana pasteurizada o leche humana pasteurizada con suplemento fortificador comercial FM85® y que no presente patologías que puedan interferir en el crecimiento y aumento de peso. A partir del análisis, se formaron dos grupos: recién nacidos pretérmino de muy bajo peso que recibieron leche humana pasteurizada (Grupo A) y los que recibieron leche humana pasteurizada suplementada con FM85® (Grupo B).
Resultados
se comprobó que no hubo diferencias estadísticamente significativas entre los recién nacidos pretérmino de muy bajo peso analizados respecto a la comparación entre los dos grupos y entre el grupo analizado en dos momentos con las diferentes dietas. Se observó que la edad gestacional influyó en el aumento de peso diario en ambas comparaciones.
Conclusión
se concluyó que las dos dietas utilizadas no implicaron diferencias estadísticamente significativas en el crecimiento cuando se compararon los dos grupos.
Recien nacido prematuro; Leche humana; Bancos de leche; Aditivos alimentarios; Enfermería
Introduction
The key principles contained in the nutrition guidelines for preterm newborns (PTNBs) include support for developmental care, breastfeeding, expressed milk, and feeding plans.11. McNelis K, Fu TT, Poindexter B. Nutrition for the Extremely Preterm Infant. Clin Perinatol. 2017;44(2):395–406. Immunological protection and intestinal maturation occurs by means of early trophic feeding with colostrum and transition milk. Studies with PTNBs show that feeding with human milk (HM) decreases the incidence of infection and necrotizing enterocolitis (NEC) in these patients and improves neurological development, however it interferes with weight gain and linear growth.11. McNelis K, Fu TT, Poindexter B. Nutrition for the Extremely Preterm Infant. Clin Perinatol. 2017;44(2):395–406.
The mean of fat in the breast milk (BM) of preterm infants’ mothers is higher at the beginning of lactation (4.32±1.01g/100mL) than in the later period (3.12±0.81g100ml). Authors state that the higher fat content in the BM of PTNBs’ mothers, especially at the beginning of lactation, seems to be physiologically important for these infants, considering that BM intake is low.22. Guerra E, Downey E, O’Mahony JA, Caboni MF, O’Shea CA, Ryan AC, et al. Influence of duration of gestation on fatty acid profiles of human milk. Eur J Lipid Sci Technol. 2016;118(11):1775–87.
Breast milk is the principal exogenous source of sialic acid (SA) for infants, which is present in oligosaccharides, glycoproteins and glycolipids. Sialic acid is essential for the synthesis of gangliosides located on the surfaces of cells of the cerebral cortex that act on the differentiation and proper functioning of nerve cells. Adequate SA supplementation rapidly increases the SA content in the cerebral cortex, which further improves learning and memory of the infants’ developing brains.33. Wang HJ, Hua CZ, Ruan LL, Hong LQ, Sheng SQ, Shang SQ. Sialic acid and iron content in breastmilk of chinese lactating women. Indian Pediatr. 2017;54(12):1029–31.
Iron concentrations in the BM of mothers of preterm and full-term infants are sufficient to restore and prevent iron deficiency anemia, which was demonstrated by a positive correlation between lactation weeks and component levels in milk (0.05 mg/L in colostrum, 0.06 mg/L in transition milk and 0.27 mg/L in mature milk).44. Boyce C, Watson M, Lazidis G, Reeve S, Dods K, Simmer K, et al. Preterm human milk composition: a systematic literature review. Br J Nutr. 2016;116(6):1033–45.However, the BM of preterm infants’ mothers contains less protein, lipids, and kilocalories than pasteurized human milk (PHM) of mothers of term infants.11. McNelis K, Fu TT, Poindexter B. Nutrition for the Extremely Preterm Infant. Clin Perinatol. 2017;44(2):395–406.
Thus, supporting HM as a food of choice for all infants is a standard practice in neonatal intensive care units (NICUs),22. Guerra E, Downey E, O’Mahony JA, Caboni MF, O’Shea CA, Ryan AC, et al. Influence of duration of gestation on fatty acid profiles of human milk. Eur J Lipid Sci Technol. 2016;118(11):1775–87.,33. Wang HJ, Hua CZ, Ruan LL, Hong LQ, Sheng SQ, Shang SQ. Sialic acid and iron content in breastmilk of chinese lactating women. Indian Pediatr. 2017;54(12):1029–31. however, it is not indicated for all the preterm infants.11. McNelis K, Fu TT, Poindexter B. Nutrition for the Extremely Preterm Infant. Clin Perinatol. 2017;44(2):395–406.
The PHM is an effective alternative source of nutrition when BM is not available, and PTNBs are the main recipients. Most of the time, this milk is obtained from mothers of healthy term infants, who donate their excess volume, which is subsequently processed and stored in human milk banks (HMB). The donated milk is pasteurized, with the objective of reducing microbial growth and guaranteeing safe consumption.11. McNelis K, Fu TT, Poindexter B. Nutrition for the Extremely Preterm Infant. Clin Perinatol. 2017;44(2):395–406.,22. Guerra E, Downey E, O’Mahony JA, Caboni MF, O’Shea CA, Ryan AC, et al. Influence of duration of gestation on fatty acid profiles of human milk. Eur J Lipid Sci Technol. 2016;118(11):1775–87.
Pasteurization causes changes in the bioactive elements of HM, with a reduction of 67-100% of immunoglobulin A (IgA) activity, and 27 - 43% reduction in the activity of lactoferrin. Lipases (lipoprotein lipase and bile salt activated lipase), which act in the digestion of triglycerides, are completely inactivated by pasteurization process.(1)
Maternal characteristics, gestational age (GA), lactation period, duration, and the method by which the milk is extracted causes variation in the macronutrient composition of HM. Thus, supplements do not consistently provide as adequate an intake of protein and calories as breastfeeding. However, protein-based supplements of bovine milk, which has a higher protein content, have provided better growth for the infant.11. McNelis K, Fu TT, Poindexter B. Nutrition for the Extremely Preterm Infant. Clin Perinatol. 2017;44(2):395–406.
Recommended intake of enteral and caloric protein for the low birth weight infants (LBWI) should be 4.0 - 4.5g/kg and 110 - 135kcal/kg/day, but the PHM composition has concentration means of 0.9 - 1.0 g/dL of protein, and between 14.6 - 19.8 kcal, demonstrating the need to supplement this nutrient.11. McNelis K, Fu TT, Poindexter B. Nutrition for the Extremely Preterm Infant. Clin Perinatol. 2017;44(2):395–406.
Thus, the main purpose of any nutritional regimen in PTNB, very-low-birth-weight infants (VLBWI) and extremely low birth weight infants (ELBWI), is to provide a growth rate similar to the intrauterine growth rate (15g/kg/day); to achieve this goal, there must be a balance between the supply of proteins and energy. As soon as the nutritional needs of these infants became known and understood, advances in diversified processes and supplemental dairy products occurred.55. Harding JE, Cormack BE, Alexander T, Alsweiler JM, Bloomfield FH. Advances in nutrition of the newborn infant. Lancet. 2017;389(10079):1660–8.,66. Adamkin DH, Radmacher PG. Fortification of human milk in very low birth weight infants (VLBW <1500 g birth weight). Clin Perinatol. 2014;41(2):405–21.
The first two weeks of the life in preterm infants, especially ELBWI, represent a critical moment for growth and nutritional quality. The initial weight loss that occurs, primarily between the fourth and ninth days of life, with a mean of five days, is the typical pattern of immediate postnatal growth. Then, beginning in the second week of life, a peak of early neonatal growth occurs, with a rate of growth that attempts to mimic intrauterine rates. Birth weight is usually recovered between eight and 24 days of age. In ELBWI newborns, the recovery is closer to 24 days of life and, in the larger infants, closer to the first days of life.77. Silveira RC, Procianoy RS. Crescimento nos primeiros anos de vida de recém-nascidos de muito baixo peso. In: Procianoy RS, Leone CR, editores. Programa de Atualização em Neonatologia (PRORN)/Sociedade Brasileira de Pediatria. Porto Alegre: Artmed/Panamericana; 2003. p. 49–86.
The most widely used measure for nutritional assessment of the newborn is weight, which is also directly related to growth. The cephalic perimeter (CP) is related to brain size and is a less sensitive indicator of malnutrition. Thus, the proportion of growth is established by relating two anthropometric factors, comparing measures that are differently affected when there is growth acceleration or deceleration. The weight/length relationship can be used to obtain the body mass index, which is a marker of adiposity that can reflect the proportionality of the growth, and can be calculated by the mean weight divided by square of the length. 88. Brock RS, Falcão MC. Nutritional assessment of newborn infants: current method limitations na new perspectives. Rev Paul Pediatr. 2008;26(1):70–6. The recovery of CP is achieved at 12 months of life.99. Rugolo LM, Bentlin MR, Rugolo Junior A, Dalben I, Trindade CE. Crescimento de prematuros de extremo baixo peso nos primeiros dois anos de vida. Rev Paul Pediatr. 2007;25(2):142–9. Therefore, the anthropometric and laboratory indicators are important in the clinical follow-up of the VLBWI.1010. Feferbaum R, Falcão MC, Schmider KR, Barros K. Recomendações nutricionais para prematuros e/ou recém-nascidos de muito baixo peso. São Paulo: International Life Science Institute do Brasil; 2016. (Série de publicações da força-tarefa de nutrição da criança, vol.1).
A commercial supplement commonly used in Brazil is the FM85®, which adds proteins, carbohydrates, vitamins, minerals, and trace elements to the PHM. Even so, there is a concern regarding PHM supplementation, as the defense action that BM provides can be modified, as well as causing intolerance within the gastrointestinal tract of the PTNB who receives supplemented HM.1111. Thomaz DM, Serafim PO, Palhares DB, Melnikov P, Venhofen L, Vargas MO. Comparação entre suplementos homólogos do leite humano e um suplemento comercial para recém-nascidos de muito baixo peso. J Pediatr (Rio J). 2012;88(2):119.,1212. Chan GM, Lee ML, Rechtman DJ. Effects of a human milk-derived human milk fortifier on the antibacterial actions of human milk. Breastfeed Med. 2007;2(4):205–8.
Therefore, the aim of this study was to compare the growth of VLBWI hospitalized in the NICU, receiving PHM and PHM enhanced with a commercial supplement.
Methods
This was an exploratory quantitative, longitudinal study of a series of cases. The population and sample consisted of all VLBWI up to 36 weeks of GA, and weight less or equal to 1,500g, who were hospitalized in the neonatal intensive care unit (NICU) of a university hospital in the northwestern part of the state of Paraná, who met met the following inclusion criteria: clinically stable, both sexes, enteral feeding by tube, receiving PHM and/or PHM enhanced with the commercial supplement FM85® (PHM+ FM85®), and without diseases (congenital malformation, chromosomal abnormalities, inborn error of metabolism; congenital infection) that could interfere with growth or weight gain. Anthropometric data were obtained until the time they received feedings via tube. The VLBWI with incomplete data were excluded from the study. The data source was the patient records.
The protocol for using the FM85® in the NICU considers that the beginning of the nutritional supplement should occur approximately 15 days after birth,66. Adamkin DH, Radmacher PG. Fortification of human milk in very low birth weight infants (VLBW <1500 g birth weight). Clin Perinatol. 2014;41(2):405–21. or as soon as the infant is receiving HM with a volume between 130 and 150mL/kg/day.
The time continuum for selection of the patient records was from 2014 to 2016, a significant time based on the number of beds, that is, six beds for intensive care and four for semi-intensive care, as well as the infants’ prolonged length of stay.
The patients that met the inclusion criteria had their medial records analyzed, and from this analysis two groups were created: VLBWI receiving PHM (Group A) and VLBWI receiving PHM + FM85® (Group B). According to the similarity between the data analyzed (GI, weight, age, diet), the cases were separated in pairs, comparing those that received PHM and PHM + FM85®.
Both groups were compared. Group A, consisting of 12 VLBWI, received PHM exclusively; Group B, composed of 14 VLBWI, initially received PHM and then, in sequence, PHM + FM85®. In the univariate analysis, the Student T-test (independent variable) was applied, and in the multivariate analysis, the generalized linear model was used.
For comparison 2, only Group B was analyzed, using the paired T-test (dependent variable) for univariate analysis; for multivariate analysis the generalized linear model with repeated measures was used.
The data identification and characterization of the NB (diagnosis, birth and corrected GA, sex, type of delivery, date of birth, days of hospitalization, caffeine and antibiotic use, use of gastric tube, incubator, mechanical ventilator, venous access, weight, cephalic perimeter, initial and final height; volume and type of milk received daily, and laboratory tests (electrolytes, calcium, phosphorus, total protein, albumin and blood count)) were entered in Microsoft Excel 2010 software, and then analyzed using the statistical software R®.
The project was approved by the Permanent Committee on Ethics in Research with Human Subjects under No. 1,922,760.
Results
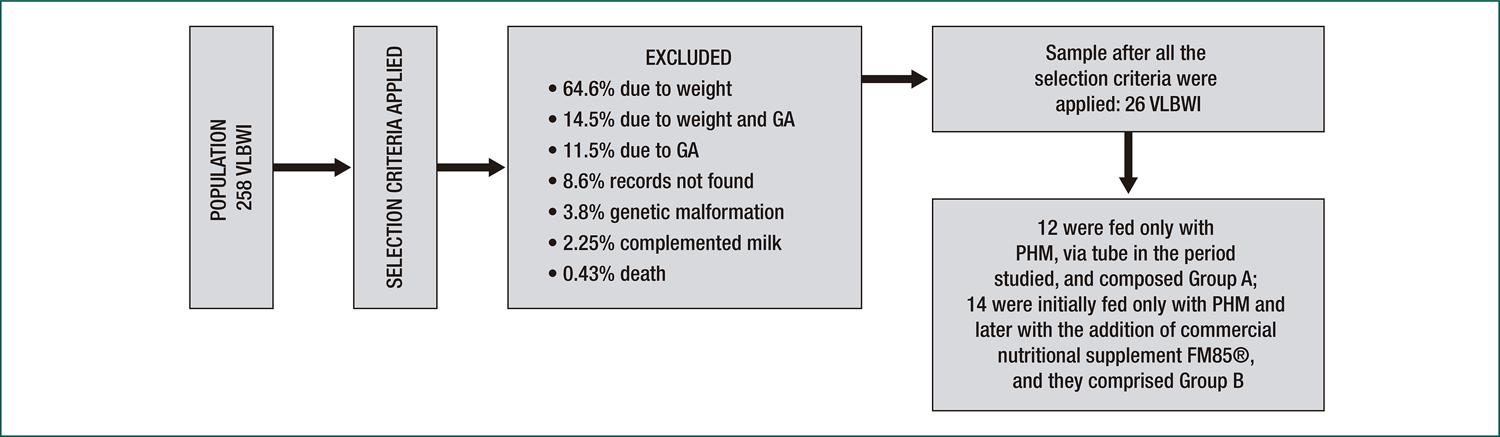
Among the 258 medical records, 232 were excluded because they did not meet the criteria defined for the research. A total of 26 records of VLBWI met the inclusion criteria. Of these, 12 were in Group A, and 14 were in Group B (Figure 1).
Diagram of the total number of charts, after application of inclusion criteria and reasons for exclusion, and the division into Groups A and B
Of the 26 VLBW premature newborns, nine were born via vaginal delivery and 17 were cesarean deliveries. The reasons for hospitalization were,: extreme prematurity, non-specific respiratory distress, hypoglycemia, hyperglycemia, early infection, neonatal sepsis, unspecified bacterial infection, bilateral inguinal hernia with obstruction without gangrene, and bronchopulmonary dysplasia originating in the perinatal period. Unspecified respiratory distress was the most prevalent pathology.
In terms of the sex, 16 newborns were male and ten were female. The GA ranged from 25 weeks (w) and four days (d) to 33 weeks. Corrected gestational age, which means the adjustment of chronological age according to the degree of prematurity, with 40 weeks as reference, was from 30w1d to 41w4d. The initial weight (at birth) varied from 665 - 1,500 grams; the height from 22 - 40cm; and, the CP from 22 - 29cm. The final weight, when the oral feeding began, ranged from 1,110 - 1,945g; the length ranged from 33.5 - 44.5cm; and, the CP from 24 - 30cm. Antimicrobial medications was used by of 92% of the sample.
The enteral feeding was performed using gastric tubes; only one infant used a nasogastric tube (NGT), while 20 newborns were fed by orogastric tube (OGT). The remaining five infants used more than one type of tube during the period of hospitalization; none developed NEC.
All newborns received parenteral nutrition for a mean of 19 days for both groups. The mean time to recover the birth weight, considering the weight loss after birth, was 12 days (SD±7.67).
Newborns of Group A had a mean GA of 29w2d, and received PHM for 18 days (SD±10.2); with a mean hospitalization of 21 days (SD±10.18), the longest length of stay was 46 days and the shortest was seven days. The mean BW was 1,319.6g (SD±97.01), and the mean final weight was 1,500g (SD±143.4). The mean length at birth in this group was 38.5 cm (SD±1.20), the longest was 40cm and the shortest was 36.5cm. Regarding CP, the mean was 27.7cm (SD±1.46) at birth and 28.1cm (SD±1.49) at the final measurement. Of the total, 42% were female and 58% were male. Regarding the type of delivery, 58% had cesarean delivery, and 42% vaginal delivery (Table 1).
Newborns of Group B, had a mean GA of 29w2d, received only PHM for a mean of 20 days (SD±8.6); and received PHM+ FM85® as an enteral diet for a mean of 17 days. The mean length of stay was 42 days (SD±15.6), the longest period was 75 days, and the shortest was 23 days. The mean BW was 1,020g (DP±224), and the mean at the final measurement was 1,484g; the mean length of this group was 35.5cm at birth and 39.8cm at the end of the study; and, the mean PC was 25.8cm at the beginning of the evaluation, and 27.8cm at the end. As for sex, 39% were female and 61% were males; in terms of type of delivery, 65% were delivered by cesarean delivery, and 35% were vaginal deliveries (Table 1).
The homogeneity of the groups can be verified regarding the sex, type of delivery, and GA of the VLBWI studied.
The univariate analysis comparing the two groups independently by means of the Student T-test established means for three variables: daily weight gain, daily length increase, and daily CP increase (Table 2).
A multivariate analysis was performed using the generalized linear model, aiming to verify if the variables (milk type, type of delivery, sex, days of hospitalization, GA, days of life for BW recovery, and days and age of onset of parenteral nutrition) did or did not affect daily weight gain, increase in length, and CP. Based on this analysis, the GA of the newborns was shown to be statistically significant in terms of weight gain. Then, a single group with two different diets (comparison 2) were compared using the repeated measures (paired) test, that is, when the newborns were fed with PHM and later with PHM + FM85® (Table 3).
No significant difference was found for these variables when compared in the two moments in the same group, but the significance value related to daily weight gain was close to the reference value. The analysis of comparisons 1 and 2 showed that, for daily weight gain, GA is a determining factor, because it reached a level of significance of less than 5%, and milk type was close to this percentage, with a significance level below than 10%.
Discussion
In a study conducted in the state of Pará, in order to trace the profile of the VLBWI (<1500g) hospitalized in the NICU, the mean length of hospitalization was 52.5 days. The researchers perceived that longer hospital stay can interfere with growth and development of newborns and can lead to an imbalance in homeostasis mechanisms and in cognitive and learning development.1313. Marcuartú AC, Malveira SS. Profile of premature newborns with very low birth weight admitted to a neonatal intensive care unit. Rev Bras Ciênc Saúde. 2017;21(1):5–10. In northern India, VLBWI were separated into two groups: one with a diet consisting of PHM enhanced with vitamins and minerals; and, the other with a supplemented PHM diet. No difference between the groups was identified, regarding the length of hospitalization.1414. Mukhopadhyay K, Narnag A, Mahajan R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: a randomized controlled trial. Indian Pediatr. 2007;44(4):286–90.
In this study, the group receiving HM (Group A) had a shorter mean hospitalization time than the group fed with PHM + FM85® (Group B), but no relationship was found between days of hospitalization (p=0.4952) or weight gain. In a randomized study with VLBWI fed exclusively with a preterm formula with bovine milk, donated and processed HM, and supplemented and processed HM, a significant difference in mean days was found using parenteral nutrition: 36 versus 27, respectively, in preterm formula made with bovine milk, versus PHM and PHM supplemented.1515. Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S. Optimizing Nutrition in Preterm Low Birth Weight Infants-Consensus Summary. Front Nutr. 2017;4:20. Our study showed that a mean of 19 days of parenteral nutrition was the same for the two groups studied, one group that received PHM and one that received PHM + FM85®. Early parenteral and enteral nutritional are recommended, to boost and improve growth and developmental outcomes in VLBWI. Parenteral nutrition is necessary at the critical moment, 11. McNelis K, Fu TT, Poindexter B. Nutrition for the Extremely Preterm Infant. Clin Perinatol. 2017;44(2):395–406.,1515. Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S. Optimizing Nutrition in Preterm Low Birth Weight Infants-Consensus Summary. Front Nutr. 2017;4:20. but the enteral nutrition is the most indicated because it helps with intestinal development, and reduces the risk of infections and sepsis. 1515. Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S. Optimizing Nutrition in Preterm Low Birth Weight Infants-Consensus Summary. Front Nutr. 2017;4:20.
The enteral feeding occurred mainly by means of orogastric tubes, because the use of a nasogastric tube is not routinely indicated, because it increases airway resistance in 30 - 50% of infants, in addition to elevating the incidence of central apnea.1515. Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S. Optimizing Nutrition in Preterm Low Birth Weight Infants-Consensus Summary. Front Nutr. 2017;4:20. Checking for gastric residue by aspirating the tube should be avoided, as it can cause gastric mucosal injury. We discuss, below, a series of studies among the feeding regimens offered in the intensive care units. In Texas (USA), they found that the PTNB infants who received supplemented PHM (p=0.03), when compared to an exclusive PHM-based diet, had a higher weight (14.0g/kg/day) and a higher growth rate (1.03cm) than infants who did not receive the supplement (12.4g/kg/day and 0.83cm) respectively.1616. Hair AB, Blanco CL, Moreira AG, Hawthorne KM, Lee ML, Rechtman DJ, et al. Randomized trial of human milk cream as a supplement to standard fortification of an exclusive human milk-based diet in infants 750-1250 g birth weight. J Pediatr. 2014;165(5):915–20.
A clinical trial with 40 VLBWI compared the weight gain among infants receiving HM with those receiving HM with additives. Infants who received HM plus an additive had a greater weight gain. None developed NEC. However, the study also suggested that, because additive nutrition was standardized and unbalanced, it could offer excess calories or insufficient amounts of protein, as it was not prepared to meet the specific needs of each premature infant.1717. Martins EC, Krebs VL. Effects of the use of fortified raw maternal milk on very low birth weight infants. J Pediatr (Rio J). 2009 Mar-Apr;85(2):157–62.
In an observational cohort study, performed in the NICU of a public maternity hospital in the city of Palmas/Tocantins, 26 PTNB were divided into two groups: 13 neonates with exclusive PHM; and 13 with PHM plus FM85®. The outcomes showed that the mean weight gain was significantly higher in the group receiving supplemented PHM. No case of NEC was identified in the study. However, in relation to length and CP, no statistically significant difference was found between the groups1818. Barbosa filho JV, Pereira RJ, Castro JGD. Efeitos do uso de fortificante do leite humano em recém-nascidos pré-termo de muito baixo peso. Ciência. Cuidado e Saúde. 2016;15(3):429–35.
Infants with VLBW, in India, were separated into two groups: one with PHM supplemented with vitamins and minerals; and another with PHM with a supplement. Those who received supplemented PHM had greater weight gain, and a greater increase in length and CP. None developed NEC.1414. Mukhopadhyay K, Narnag A, Mahajan R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: a randomized controlled trial. Indian Pediatr. 2007;44(4):286–90.
Another randomized, double blind, controlled trial was performed with VLBWI, in which one group received HM (from the mother or HM bank) supplemented with 5% FM85® (n= 4) and the other received PreNAN® 19%, diluted volume by volume with PHM (n=11). All the participants received vitamin supplements from the eighth day of life, and iron from the 16th day. Among the results, the weight gain rate was lower in the group with FM85® compared to those who received Pre Nan® as an additive. The serum alkaline phosphatase level was higher in the FM85® group than in the PreNAN® group, and there was no statistically significant difference in serum calcium and phosphorus levels. All values were considered within the normal range, and no premature newborn showed radiological signs of demineralization. There were no cases of NEC. 1616. Hair AB, Blanco CL, Moreira AG, Hawthorne KM, Lee ML, Rechtman DJ, et al. Randomized trial of human milk cream as a supplement to standard fortification of an exclusive human milk-based diet in infants 750-1250 g birth weight. J Pediatr. 2014;165(5):915–20.
One study compared an HM fortification scheme with fixed amounts of a multicomponent additive (FM85® - 5%) and a adjusted schedule, based on the serum urea concentrations measured twice weekly (FM 85® 2.5%, 5% , 6.25%, 6.25% + 0.4g protein and 6.25% + 0.8g protein). The hypothesis investigated was that PTNB fed with the adjustable regimen would present a higher protein intake, and better weight gain, than those receiving standard HM fortification scheme. It was a controlled clinical trial, blinded to the caregiver (but not to the investigator), in which the preterm infants weighing at birth between 600 and 1750g and with a GA at birth between 26 - 34 weeks were enrolled. The randomization was stratified by birth weight (≤ 1,250g, 1,251 - 1,500g, 1,501 - 1,750g) and infants received fresh or frozen mother’s HM or bank HM. The infants who received the adjustable fortification regimen had significantly greater gains in weight and CP than infants who received standard supplementation. No baby developed enterocolitis or any systemic disease. The researchers concluded that increased protein intake would have been primarily responsible for growth in the adjustable regimen.1919. Arslanoglu S, Moro GE, Ziegler EE. Adjustable fortification of human milk fed to preterm infants: does it make a difference? J Perinatol. 2006;26(10):614–21.
In this study, no statistically significant difference was identified in the anthropometric measurements between the VLBWI who received PHM and those who received PHM + FM85® for daily weight gain (p=0.7971), length increase (p=0.1806), and CP increase (p=0.1940). A similar result was obtained when comparing the use of the two diets in the same group (comparison 2). Cases of NEC were non-existent in this investigation. Serum alkaline phosphatase levels could not be analyzed, because not all of these data were recorded in the medical records. Studies have indicated that infants who received supplemented PHM had higher anthropometric indices than infants who did not receive supplemented milk. And, no infants developed NEC due to supplementation.1414. Mukhopadhyay K, Narnag A, Mahajan R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: a randomized controlled trial. Indian Pediatr. 2007;44(4):286–90.,1919. Arslanoglu S, Moro GE, Ziegler EE. Adjustable fortification of human milk fed to preterm infants: does it make a difference? J Perinatol. 2006;26(10):614–21.
Although HM supplementation is useful for VLBWI, sometimes standard supplementation may not provide the protein requirement recommended for these infants. To overcome this limitation and to optimize HM supplementation, individualized supplementation should be used. 1515. Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S. Optimizing Nutrition in Preterm Low Birth Weight Infants-Consensus Summary. Front Nutr. 2017;4:20.,2020. Brown JV, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst Rev. 2016;(5):CD000343.Another limiting factor to be considered is the cost of the product.1414. Mukhopadhyay K, Narnag A, Mahajan R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: a randomized controlled trial. Indian Pediatr. 2007;44(4):286–90. Rapid weight gain in premature babies can trigger cardiovascular risk in the future. Thus, ambulatory follow-up is important, to monitor body weight, preferably until adolescence. 1515. Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S. Optimizing Nutrition in Preterm Low Birth Weight Infants-Consensus Summary. Front Nutr. 2017;4:20. Fortification can be particularly useful in infants who were born small for gestational age, as cost remains an important limiting factor in the use of fortifier (INDIA).
Some limitations of the study were the reduced number of VLBWI, despite the three-year interval, working with data and anthropometric measures described in medical records, which do not have the research mark. However, the study can contribute to the research conducted in the area, as it has produced support that confirms that the diet with PHM+ FM85® does not imply statistically significant differences in the anthropometric measurements of VLBWI.
Conclusion
The two diets used in this study did not imply a significant difference in the anthropometric measures evaluated in the VLBWI when compared in both groups, and the two diets in the same group. Gestational age was the variable that influenced daily weight gain in both comparisons.
Referências
-
1McNelis K, Fu TT, Poindexter B. Nutrition for the Extremely Preterm Infant. Clin Perinatol. 2017;44(2):395–406.
-
2Guerra E, Downey E, O’Mahony JA, Caboni MF, O’Shea CA, Ryan AC, et al. Influence of duration of gestation on fatty acid profiles of human milk. Eur J Lipid Sci Technol. 2016;118(11):1775–87.
-
3Wang HJ, Hua CZ, Ruan LL, Hong LQ, Sheng SQ, Shang SQ. Sialic acid and iron content in breastmilk of chinese lactating women. Indian Pediatr. 2017;54(12):1029–31.
-
4Boyce C, Watson M, Lazidis G, Reeve S, Dods K, Simmer K, et al. Preterm human milk composition: a systematic literature review. Br J Nutr. 2016;116(6):1033–45.
-
5Harding JE, Cormack BE, Alexander T, Alsweiler JM, Bloomfield FH. Advances in nutrition of the newborn infant. Lancet. 2017;389(10079):1660–8.
-
6Adamkin DH, Radmacher PG. Fortification of human milk in very low birth weight infants (VLBW <1500 g birth weight). Clin Perinatol. 2014;41(2):405–21.
-
7Silveira RC, Procianoy RS. Crescimento nos primeiros anos de vida de recém-nascidos de muito baixo peso. In: Procianoy RS, Leone CR, editores. Programa de Atualização em Neonatologia (PRORN)/Sociedade Brasileira de Pediatria. Porto Alegre: Artmed/Panamericana; 2003. p. 49–86.
-
8Brock RS, Falcão MC. Nutritional assessment of newborn infants: current method limitations na new perspectives. Rev Paul Pediatr. 2008;26(1):70–6.
-
9Rugolo LM, Bentlin MR, Rugolo Junior A, Dalben I, Trindade CE. Crescimento de prematuros de extremo baixo peso nos primeiros dois anos de vida. Rev Paul Pediatr. 2007;25(2):142–9.
-
10Feferbaum R, Falcão MC, Schmider KR, Barros K. Recomendações nutricionais para prematuros e/ou recém-nascidos de muito baixo peso. São Paulo: International Life Science Institute do Brasil; 2016. (Série de publicações da força-tarefa de nutrição da criança, vol.1).
-
11Thomaz DM, Serafim PO, Palhares DB, Melnikov P, Venhofen L, Vargas MO. Comparação entre suplementos homólogos do leite humano e um suplemento comercial para recém-nascidos de muito baixo peso. J Pediatr (Rio J). 2012;88(2):119.
-
12Chan GM, Lee ML, Rechtman DJ. Effects of a human milk-derived human milk fortifier on the antibacterial actions of human milk. Breastfeed Med. 2007;2(4):205–8.
-
13Marcuartú AC, Malveira SS. Profile of premature newborns with very low birth weight admitted to a neonatal intensive care unit. Rev Bras Ciênc Saúde. 2017;21(1):5–10.
-
14Mukhopadhyay K, Narnag A, Mahajan R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: a randomized controlled trial. Indian Pediatr. 2007;44(4):286–90.
-
15Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S. Optimizing Nutrition in Preterm Low Birth Weight Infants-Consensus Summary. Front Nutr. 2017;4:20.
-
16Hair AB, Blanco CL, Moreira AG, Hawthorne KM, Lee ML, Rechtman DJ, et al. Randomized trial of human milk cream as a supplement to standard fortification of an exclusive human milk-based diet in infants 750-1250 g birth weight. J Pediatr. 2014;165(5):915–20.
-
17Martins EC, Krebs VL. Effects of the use of fortified raw maternal milk on very low birth weight infants. J Pediatr (Rio J). 2009 Mar-Apr;85(2):157–62.
-
18Barbosa filho JV, Pereira RJ, Castro JGD. Efeitos do uso de fortificante do leite humano em recém-nascidos pré-termo de muito baixo peso. Ciência. Cuidado e Saúde. 2016;15(3):429–35.
-
19Arslanoglu S, Moro GE, Ziegler EE. Adjustable fortification of human milk fed to preterm infants: does it make a difference? J Perinatol. 2006;26(10):614–21.
-
20Brown JV, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst Rev. 2016;(5):CD000343.
Publication Dates
-
Publication in this collection
10 Oct 2019 -
Date of issue
Sep-Oct 2019
History
-
Received
14 Jan 2019 -
Accepted
21 May 2019